Making point-of-care diagnostics possible

To meet the demand for point-of-care (POC) COVID-19 testing, a team from Fast Radius and the University of Illinois used additive manufacturing to create a validated, design-patented prototype of a microfluidic diagnostic in a matter of weeks, saving years of development time compared to traditionally-made biomedical diagnostics.
The challenge
Compressing a years-long product development cycle into weeks
COVID-19 tests are critical to the reopening of businesses and public spaces across the United States, but hospitals and lab facilities don’t have the capacity to handle the millions of tests needed every day.
To fill the gap, companies are scrambling to produce fast, accurate, point-of-care (POC) diagnostics that don’t need to be processed by skilled technicians.
Microfluidic “lab on a chip” technology seems like a good candidate for portable, easy to use POC COVID-19 testing, but these diagnostics usually take many years to develop, and the microfluidic cartridges are prohibitively difficult and costly to manufacture.
Two years before the pandemic, researchers at Fast Radius and the University of Illinois realized that making microfluidic cartridges with additive manufacturing could dramatically accelerate diagnostics R&D and also save time and money in production.
Once the coronavirus pandemic hit, the team quickly shifted focus to COVID-19 testing, producing a functional lab test in just five weeks.

Additive manufacturing allowed us to make significant progress with microfluidic diagnostics very quickly to address the need for POC testing. Our microfluidic cartridge is ready for additive production after only five weeks of development.
making it possible
Fast, flexible product development
1. Sophisticated technology and materials
Fast Radius has the advanced technology and materials expertise needed to make sensitive medical diagnostics. We were able to carefully control the manufacturing process to achieve the high resolution and tolerances required for microfluidic devices. Further, we made the devices with a polyurethane that has the chemical stability necessary to conduct diagnostic chemistry with precision.

2. Design flexibility and improved functionality
We designed the microfluidic cartridge so that the test sample and other chemicals can be loaded onto the device more easily than other cartridges on the market. Injection molding only allows for mixing channels to sit on the surface of the cartridge, but additive manufacturing enabled us to add an internal serpentine channel that allows fluid to move from the front to the back of the chip, resulting in better mixing performance than a 2D serpentine chip at less than half the size.
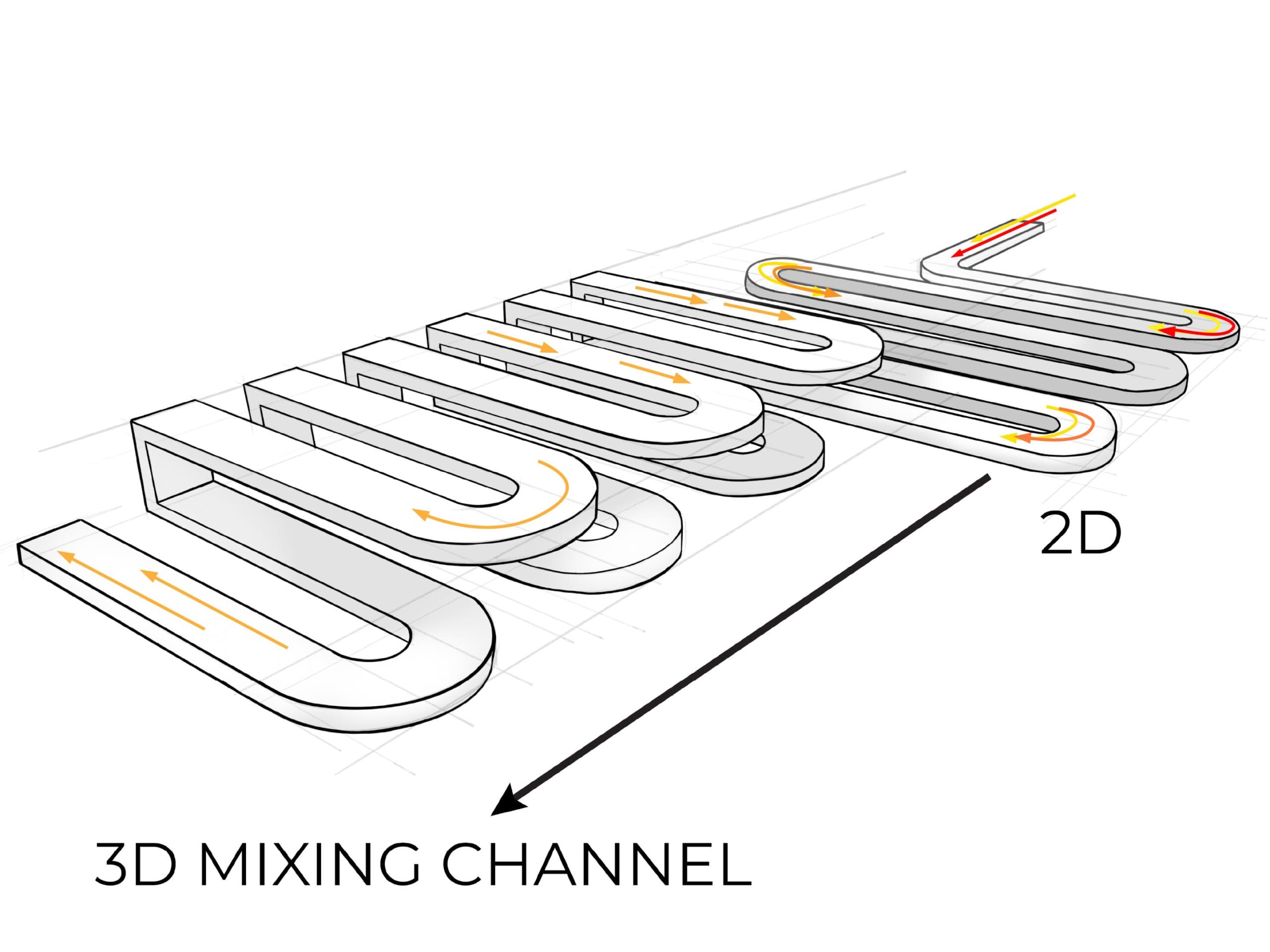
3. Rapid product development
Microfluidic diagnostics devices can take years to get to market because manufacturing the tiny cartridges is a painstaking process. The patented, additively manufactured microfluidics device from Fast Radius was designed and validated in only five weeks. Engineers worked to design, print, and test around 10 iterations of the diagnostic in four 24-hour cycles, with no need to design and test costly tooling.

4. Production-ready cost savings
Unlike a traditionally made microfluidic cartridge, the test device we manufactured can go into production with no further changes to its materials or design. Since it doesn’t require the steep upfront expenditure on micro injection molding tooling, making a diagnostic with the process we developed could save companies millions of dollars over injection molding.

Gallery




the results
Quicker, more efficient point of care diagnostics made possible with additive manufacturing
2x
Smaller than a 2D cartridge
5 weeks
From idea to working diagnostic
10 designs
Tested over four 24-hour design, test, print cycles

 Back
Back





